what did scientist study to make the first periodic table
Every field of science has its favorite ceremony.
For physics, information technology's Newton'southward Principia of 1687, the volume that introduced the laws of motion and gravity. Biology celebrates Darwin's On the Origin of Species (1859) forth with his birthday (1809). Astronomy fans commemorate 1543, when Copernicus placed the sunday at the center of the solar system.
And for chemistry, no cause for commemoration surpasses the origin of the periodic tabular array of the elements, created 150 years agone this March by the Russian chemist Dmitrii Ivanovich Mendeleev.
Mendeleev's table has go as familiar to chemistry students as spreadsheets are to accountants. It summarizes an entire scientific discipline in 100 or then squares containing symbols and numbers. It enumerates the elements that compose all earthly substances, bundled so every bit to reveal patterns in their properties, guiding the pursuit of chemical research both in theory and in practice.
"The periodic table," wrote the chemist Peter Atkins, "is arguably the most important concept in chemistry."
Mendeleev'due south table looked like an ad hoc nautical chart, but he intended the tabular array to limited a deep scientific truth he had uncovered: the periodic constabulary. His law revealed profound familial relationships among the known chemic elements — they exhibited similar backdrop at regular intervals (or periods) when arranged in lodge of their atomic weights — and enabled Mendeleev to predict the beingness of elements that had not yet been discovered.
"Before the promulgation of this constabulary the chemical elements were mere fragmentary, incidental facts in Nature," Mendeleev declared. "The law of periodicity first enabled united states to perceive undiscovered elements at a distance which formerly was inaccessible to chemical vision."
Mendeleev's table did more than than foretell the existence of new elements. It validated the then-controversial conventionalities in the reality of atoms. Information technology hinted at the existence of subatomic structure and anticipated the mathematical appliance underlying the rules governing matter that somewhen revealed itself in breakthrough theory. His table finished the transformation of chemical science from the medieval magical mysticism of abracadabra to the realm of modern scientific rigor. The periodic table symbolizes not just the constituents of matter, but the logical cogency and principled rationality of all science.
Laying the groundwork
Legend has it that Mendeleev conceived and created his table in a unmarried twenty-four hours: Feb 17, 1869, on the Russian calendar (March i in most of the rest of the world). But that's probably an exaggeration. Mendeleev had been thinking about grouping the elements for years, and other chemists had considered the notion of relationships among the elements several times in the preceding decades.
In fact, German chemist Johann Wolfgang Döbereiner noticed peculiarities in groupings of elements as early on equally 1817. In those days, chemists hadn't yet fully grasped the nature of atoms, as described in the atomic theory proposed by English schoolteacher John Dalton in 1808. In his New Organization of Chemic Philosophy, Dalton explained chemical reactions by assuming that each simple substance was fabricated of a particular blazon of atom.
Chemic reactions, Dalton proposed, produced new substances when atoms were disconnected or joined. Whatever given chemical element consisted entirely of 1 kind of cantlet, he reasoned, distinguished from other kinds by weight. Oxygen atoms weighed 8 times as much as hydrogen atoms; carbon atoms were six times as heavy equally hydrogen, Dalton believed. When elements combined to brand new substances, the amounts that reacted could be calculated with cognition of those atomic weights.
Dalton was incorrect about some of the weights — oxygen is really sixteen times the weight of hydrogen, and carbon is 12 times heavier than hydrogen. Simply his theory fabricated the thought of atoms useful, inspiring a revolution in chemistry. Measuring atomic weights accurately became a prime number preoccupation for chemists in the decades that followed.
When contemplating those weights, Döbereiner noted that certain sets of three elements (he chosen them triads) showed a peculiar relationship. Bromine, for example, had an atomic weight midway betwixt the weights of chlorine and iodine, and all 3 elements exhibited similar chemical behavior. Lithium, sodium and potassium were also a triad.
Other chemists perceived links between atomic weights and chemical properties, simply it was not until the 1860s that atomic weights had been well enough understood and measured for deeper insights to emerge. In England, the chemist John Newlands noticed that arranging the known elements in lodge of increasing atomic weight produced a recurrence of chemical properties every eighth element, a pattern he called the "law of octaves" in an 1865 paper. But Newlands' blueprint did non concord up very well subsequently the first couple of octaves, leading a critic to advise that he should try arranging the elements in alphabetical order instead. Clearly, the relationship of element properties and atomic weights was a bit more than complicated, as Mendeleev soon realized.
Organizing the elements
Born in Tobolsk, in Siberia, in 1834 (his parents' 17th child), Mendeleev lived a dispersed life, pursuing multiple interests and traveling a higgledy-piggledy path to prominence. During his college instruction at a pedagogy constitute in Leningrad, he nearly died from a serious affliction. Afterward graduation, he taught at eye schools (a requirement of his scholarship at the didactics constitute), and while pedagogy math and science, he conducted inquiry for his principal's caste.
He then worked as a tutor and lecturer (along with some pop science writing on the side) until earning a fellowship for an extended tour of research at Europe'southward most prominent university chemistry laboratories.
When he returned to St. petersburg, he had no task, so he wrote a masterful handbook on organic chemical science in hopes of winning a big cash prize. It was a long shot that paid off, with the lucrative Demidov Prize in 1862. He also found work as an editor, translator and consultant to various chemical industries. Eventually he returned to research, earning his Ph.D. in 1865 and then becoming a professor at the University of Saint petersburg.
Soon thereafter, Mendeleev found himself about to teach inorganic chemical science. In preparing to master that new (to him) field, he was unimpressed by the available textbooks. So he decided to write his own. Organizing the text required organizing the elements, so the question of how best to arrange them was on his mind.
By early 1869, Mendeleev had made enough progress to realize that some groups of like elements showed a regular increase in atomic weights; other elements with roughly equal diminutive weights shared common backdrop. It appeared that ordering the elements by their atomic weight was the cardinal to categorizing them.
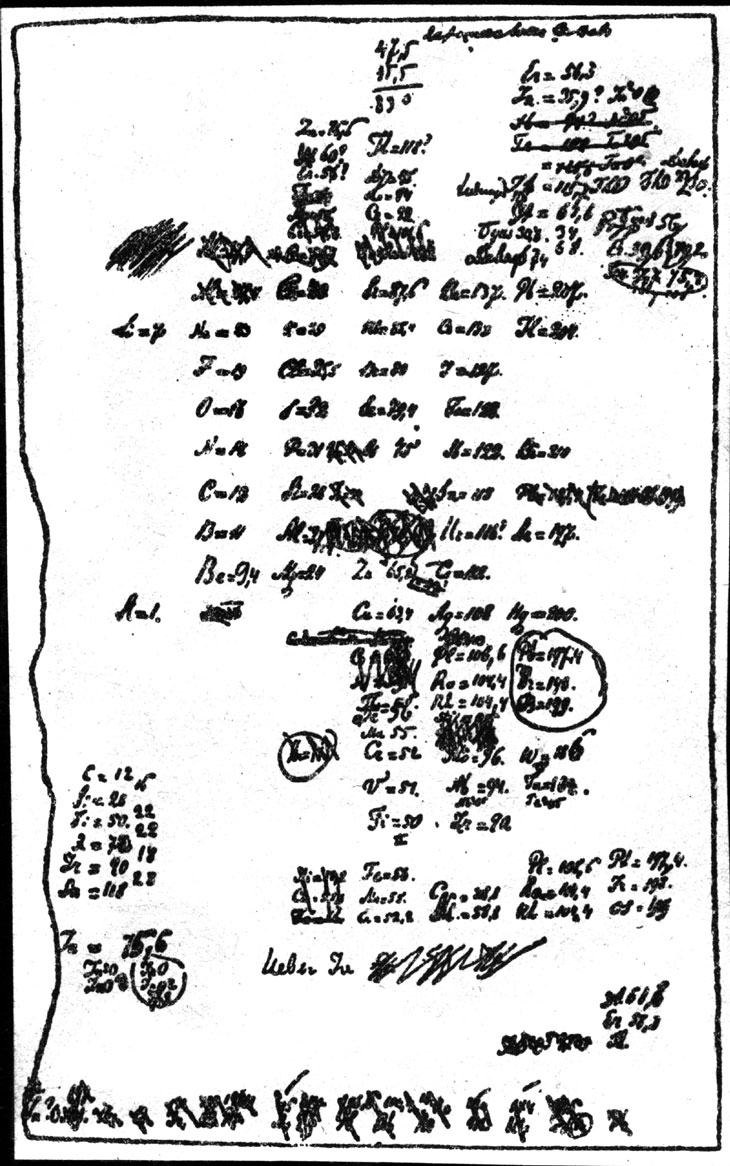
Past Mendeleev'due south own account, he structured his thinking past writing each of the 63 known elements' properties on an individual note menu. Then, by way of a sort of game of chemic solitaire, he establish the pattern he was seeking. Arranging the cards in vertical columns from lower to higher atomic weights placed elements with like backdrop in each horizontal row. Mendeleev's periodic table was born. He sketched out his tabular array on March i, sent it to the printer and incorporated information technology into his before long-to-be-published textbook. He quickly prepared a paper to exist presented to the Russian Chemic Society.
"Elements arranged according to the size of their atomic weights show clear periodic properties," Mendeleev declared in his paper. "All the comparisons which I have made … lead me to conclude that the size of the diminutive weight determines the nature of the elements."
Meanwhile, the German chemist Lothar Meyer had also been working on organizing the elements. He prepared a table similar to Mendeleev'due south, perhaps fifty-fifty earlier Mendeleev did. But Mendeleev published first.
More important than beating Meyer to the publication punch, though, was Mendeleev'due south utilize of his table to make bold predictions about undiscovered elements. In preparing his table, Mendeleev had noticed that some note cards were missing. He had to leave blank spaces to go the known elements to properly marshal. Within his lifetime, 3 of those blanks were filled with the previously unknown elements gallium, scandium and germanium.
Not just had Mendeleev predicted the existence of these elements, but he had also correctly described their properties in item. Gallium, for instance, discovered in 1875, had an atomic weight (every bit measured then) of 69.nine and a density six times that of water. Mendeleev had predicted an chemical element (he chosen it eka-aluminum) with just that density and an atomic weight of 68. His predictions for eka-silicon closely matched germanium (discovered in 1886) in atomic weight (72 predicted, 72.3 observed) and density (v.5 versus 5.469). He besides correctly predicted the density of germanium's compounds with oxygen and chlorine.
Mendeleev's table had get an oracle. Information technology was every bit if end-of-game Scrabble tiles spelled out the secrets of the universe. While others had glimpsed the periodic law's power, Mendeleev was the master at exploiting it.
Mendeleev's successful predictions earned him legendary condition as a maestro of chemic wizardry. But today, historians dispute whether the discovery of the predicted elements cemented the acceptance of his periodic law. The law'south approval may take been more due to its power to explain established chemic relationships. In whatever case, Mendeleev's prognosticative accurateness certainly attracted attention to the merits of his table.
By the 1890s, chemists widely recognized his constabulary as a landmark in chemic noesis. In 1900, the future Nobel chemistry laureate William Ramsay called information technology "the greatest generalization which has as yet been made in chemistry." And Mendeleev had done it without agreement in any deep way why it worked at all.
A mathematical map
In many instances in the history of science, grand predictions based on novel equations have turned out to be right. Somehow math reveals some of nature's secrets before experimenters discover them. Antimatter is one example, the expansion of the universe another. In Mendeleev'due south instance, the predictions of new elements emerged without any artistic mathematics. But in fact, Mendeleev had discovered a deep mathematical map of nature, for his tabular array reflected the implications of breakthrough mechanics, the mathematical rules governing atomic architecture.
In his textbook, Mendeleev had noted that "internal differences of the matter that comprises the atoms" could be responsible for the elements' periodically recurring properties. But he did not pursue that line of idea. In fact, over the years he waffled near how important atomic theory was for his table.
But others could read the table'southward message. In 1888, High german pharmacist Johannes Wislicenus declared that the periodicity of the elements' properties when arranged by weight indicated that atoms are composed of regular arrangements of smaller particles. So in a sense, Mendeleev'south tabular array did anticipate (and provide bear witness for) the complex internal structure of atoms, at a fourth dimension when nobody had any idea what an atom really looked like, or even whether information technology had any internal structure at all.
By the time of Mendeleev'southward death in 1907, scientists knew that atoms had parts: electrons, which carried a negative electric charge, plus some positively charged component to make atoms electrically neutral. A central clue to how those parts were arranged came in 1911, when the physicist Ernest Rutherford, working at the Academy of Manchester in England, discovered the atomic nucleus. Before long thereafter Henry Moseley, a physicist who had worked with Rutherford, demonstrated that the amount of positive charge in the nucleus (the number of protons it independent, or its "atomic number") determined the correct order of the elements in the periodic table.
Atomic weight was closely related to Moseley's atomic number — close enough that ordering elements by weight differs in only a few spots from ordering by number. Mendeleev had insisted that those weights were wrong and needed to be remeasured, and in some cases he was right. A few discrepancies remained, just Moseley's atomic number gear up the tabular array straight.
At about the same fourth dimension, the Danish physicist Niels Bohr realized that quantum theory governed the arrangement of electrons surrounding the nucleus and that the outermost electrons adamant an element'south chemic properties.

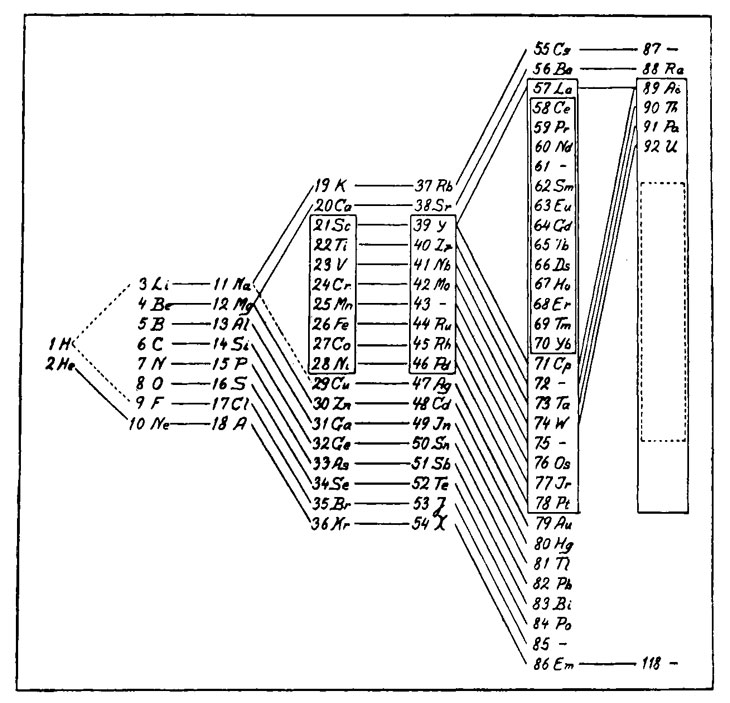
Similar arrangements of the outer electrons would recur periodically, explaining the patterns that Mendeleev's tabular array had originally revealed. Bohr created his ain version of the tabular array in 1922, based on experimental measurements of electron energies (along with some guidance from the periodic law).
Bohr's table added elements discovered since 1869, just it was still, in essence, the periodic arrangement that Mendeleev had discovered. Without the slightest inkling to quantum theory, Mendeleev had created a table reflecting the atomic compages that breakthrough physics dictated.
Bohr's new table was neither the first nor final variant on Mendeleev's original blueprint. Hundreds of versions of the periodic tabular array take been devised and published. The modern form, a horizontal design in contrast with Mendeleev's original vertical version, became widely pop only after World War II, largely due to the work of the American chemist Glenn Seaborg (a longtime member of the board of Science Service, the original publisher of Science News).
Seaborg and collaborators had synthetically produced several new elements with atomic numbers across uranium, the last naturally occurring element in the tabular array. Seaborg saw that these elements, the transuranics (plus the 3 elements preceding uranium) demanded a new row in the table, something Mendeleev had not foreseen. Seaborg'south table added the row for those elements beneath a similar row for the rare earth elements, whose proper place had never been quite clear, either. "Information technology took a lot of guts to buck Mendeleev," Seaborg, who died in 1999, said in a 1997 interview.
Seaborg's contributions to chemistry earned him the honor of his own namesake chemical element, seaborgium, number 106. It'southward one of a handful of elements named to honor a famous scientist, a list that includes, of course, element 101, discovered by Seaborg and colleagues in 1955 and named mendelevium — for the chemist who above all others deserved a place at the periodic table.

Source: https://www.sciencenews.org/article/periodic-table-history-chemical-elements-150-anniversary

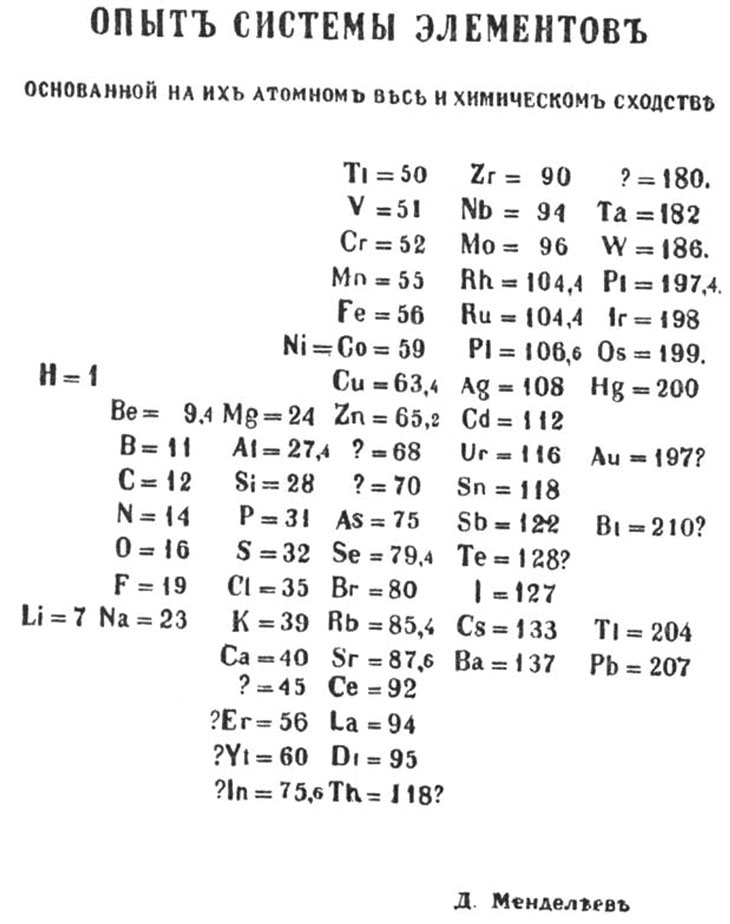
Post a Comment for "what did scientist study to make the first periodic table"